This article is the first in our series on advancements in medical isolation transformer design.
Medical devices form an important component of patient care. Their complexities are coupled with the presence of power factors in most medical devices, thus leading to heightened safety standards and procedures.
What Is a Medical Device?
A medical device may be defined as any appliance, instrument, material, apparatus or other article, either used in a singular form in combination with other equipment/devices, including the software essential for its intended purpose by the manufacturer to be used for human beings for the following purpose of:
- diagnosis, prevention, monitoring, treatment or alleviation of disease
- diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap
- investigation, replacement or modification of the anatomy or of a physiological process
- control of conception and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.
Medical devices form an important component of patient care. From tongue depressors to dialysis machines, medical devices encompass a very broad and complex variety of technologies. The complexities are coupled with the presence of power factors in most medical devices. Thus, in addition to the device performance, the crucial aspect of patient safety and the health provider’s safety gets incorporated. In order to comply with all safety requirements, sets of universal standards and norms have been prescribed, compliance to which ensures delivery of the right technology in the right way. A means to verify the devices against this desired compliance is testing. Thus, product testing brings into existence the first level of assessment of appropriateness and safety of a device.
With developing economies and increasing awareness, people are becoming more conscious about their health. Regardless of the cost factor, people are willing to opt for advanced technologies and solutions to improve their health. Hence medical devices have seen significant growth in the healthcare industry. Further, the medical device industry has its sub-industries like diagnostics, imaging, cardiovascular devices, surgical devices, and orthopedic devices.
Medical Device Types
Active medical device — any medical device relying for its functioning on a source of electrical energy or any source of power other than that directly generated by the human body or gravity.
Active implantable medical device — any active medical device which is intended to be totally or partially introduced, surgically or medically, into the human body or by medical intervention into a natural orifice, and which is intended to remain after the procedure.
In vitro diagnostic medical device — any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, equipment, or system, whether used alone or in combination intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information:
- concerning a physiological or pathological state, or
- concerning a congenital abnormality, or
- to determine the safety and compatibility with potential recipients, or
- to monitor therapeutic measures.
Medical Device Classification
| Class | Characterization / Device type | Example |
| Class I | Low risk level | Thermometers, Tongue depressors |
| Class IIA | Low to Moderate risk level | Hypodermic needles |
| Class IIB | Moderate to High risk level | Lung ventilators and bone fixation plates |
| Class III | High risk level | Heart valves and implantable defibrillators |
Environment, destination and intended use are determinant for the classification of a medical device.
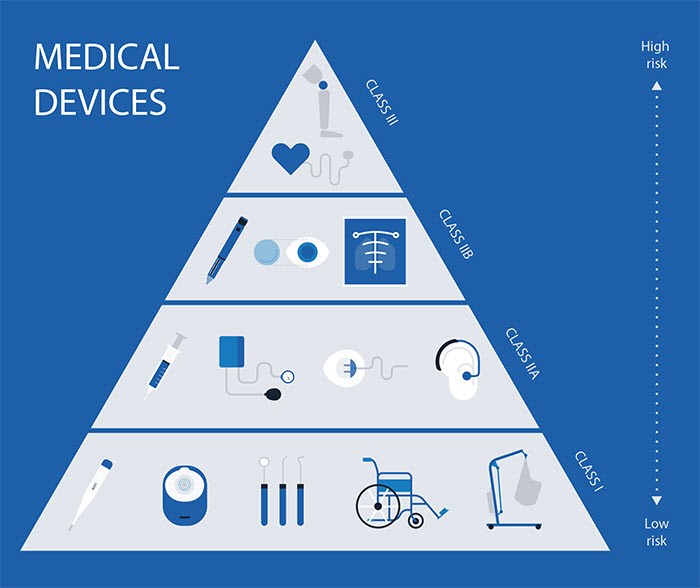
Image source: https://towardsdatascience.com/how-to-get-clinical-ai-tech-approved-by-regulators-fa16dfa1983b
Examples of Medical Devices

Image source: http://higportfolio.com/ace/www/medical_springs.html
In our next article, we look more closely at medical devices that require electricity to function and examine their risk factors, classifications, and safety standards.
Learn more in our white paper — Improving Medical Isolation Transformers with Segmented Core Cap Technology