Medical Electrical Equipment Safety and Regulation.
Medical equipment can expose patients and caregivers to potential electrical hazards. Several international standards exist to ensure adequate protection is incorporated into equipment designs.
This article is the second in our series on advancements in medical isolation transformer design.
What Is Medical Electrical Equipment?
Medical electrical equipment is provided with no more than one connection to a particular mains supply and is intended to:
- diagnose the patient
- treat the patient
- monitor the patient under medical supervision
- make physical or electrical contact with the patient
- transfer energy to or from the patient and/or detect such energy transfer
to or from the patient
The equipment includes those accessories as defined by the manufacturer which are necessary to enable the normal use of the equipment.
During normal operation and in the event of a malfunction, it is imperative that the equipment does not pose any danger to patients or medical staff. A piece of equipment that causes a short circuit or residual current can trigger a protective system upstream and in doing so shut down other, possibly life-sustaining, equipment. Thus, it is necessary to pay special attention to how each unit is supplied with power.
The use of electricity for medical diagnostic, measurement and therapy equipment potentially exposes patients and even caregivers to the risk of electrical shock, burns, internal organ damage, and cardiac arrhythmias directly due to corriente de fuga resulting from improper grounding and electrical isolation. The electrical conductivity of body fluids and the presence of various conductive solutions and gels in the patient care system make this environment even more vulnerable.
Several techniques commonly provide isolation when designing electronic equipment. Careful component placement and printed-circuit-board layout can provide adequate room for creepage and clearance of components in close proximity of high voltages. Meeting these two specifications can be a tedious, time-consuming task, and each component subject to them must meet the requirements called out in the applicable standards.
Medical Equipment Regulation
Most major world markets regulate medical equipment. In the United States, the Federal Food, Drug and Cosmetic Act (and succeeding acts) requires that all medical devices be “safe and effective,” and FDA recognizes consensus standards as a means to support a declaration of conformity (new 510(k) paradigm, “abbreviated 510(k)”). FDA lists IEC 60601 + national deviations (UL 2601-1) as a recognized consensus standard.
In Europe, the Medical Devices Directive (93/42/EEC, Article 3) requires medical devices to meet the “essential requirements.” Compliance is presumed by conformity to the harmonized standards in the Official Journal of the EC (93/42/EEC, Article 5). IEC 60601 + regional deviations (EN 60601) is a harmonized standard. Similarly, IEC 60601 forms the basis for national medical equipment safety standards in many countries, including Japan, Canada, Brazil, Australia, and South Korea.
IEC 60601-1 Overview
IEC 60601 is a series of technical standards for the safety and effectiveness of medical electrical equipment.
The primary standard governing medical device design is formally known as IEC 60601-1 – Medical electrical equipment – Part 1: General requirements for basic safety and essential performance. More simply it is referred to as IEC 60601-1 or just “60601,” and compliance with this standard has become a de facto requirement for bringing new medical devices to market in many countries.
We will look at the global adoption of the standard in more detail later, but it is worth noting that there are European (EN 60601-1) and Canadian (CSA 60601-1) versions of the standard that are identical to the IEC standard. There are also deviations from the standard that relate to country-specific requirements.
Within IEC 60601-1, there are “collateral” standards that are denoted as IEC 60601-1-x; for example, IEC 60601-1-2 is the EMC collateral standard mentioned above. Other collateral standards include 60601-1-3, covering radiation protection for diagnostic x-ray systems, 60601-1-9 relating to environmental design, and 60601-1-11 recently introduced for home healthcare equipment.
As well as collateral standards, there are also many “particular” standards, denoted as IEC 60601-2-x that define specific requirements related to particular types of products, e.g. 60601-2-16 covers blood dialysis and filtration equipment.
Medical Electrical Equipment Classification
Applied parts (AP) is a part of medical electrical equipment that in normal use necessarily comes into physical contact with the patient for medical electrical equipment or a medical electrical system to perform its function.
There are three classification types of applied parts:
Type B (Body) – No electrical contact with patient and maybe earthed
Type BF (Body Floating) – Electrically connected to patient but not directly to heart
Type CF (Cardiac floating) – Electrically connected to the heart of the patient
| Parameters | Type B | Type BF | Type CF |
| Symbol as per IEC/EN 60601-1 |  |  |  |
| Examples | LED operating lighting, medical lasers, MRI body scanners, hospital beds and phototherapy equipment | Blood pressure monitors, incubators and ultrasound equipment | Dialysis machines |
The purpose of safety testing medical electronic equipment is to ensure that a device is safe from electrical hazards to patients, maintenance personnel and users.
Electric shock is caused by electricity flowing through the body after touching a damaged electrical device and results muscle spasms, burns, cardiac and respiratory arrest, and ventricular fibrillation.
Electrical Safety
Electrical safety in a hospital is a shared responsibility between several parties including:
- physicians
- nurses
- engineers (electrical, biomedical, facility, etc)
- manufacturers
The increasing number of medical devices being used in hospitals creates several basic concerns, one of which is patient safety. An important electrical safety requirement is to measure the leakage current.
Safety Classifications in Medical Standards
MOPP (Means of Patient Protection) — Electrical equipment with direct patient contact must fulfill the highest safety requirements.
MOOP (Means of Operator Protection) — Electrical equipment without direct patient contact must fulfill high safety requirements.
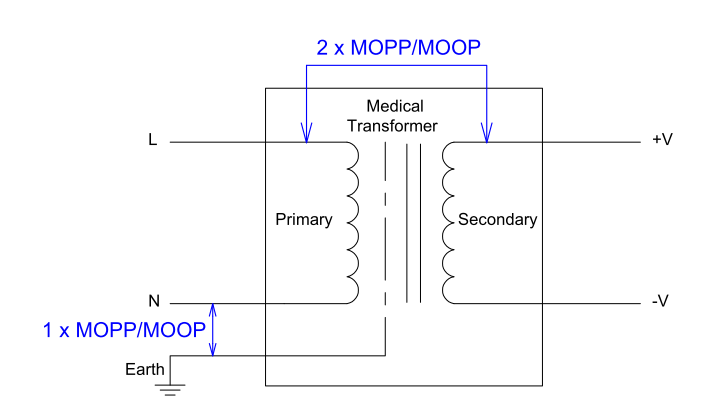
It is the responsibility of the medical product manufacturer to determine the likelihood of a patient coming into contact with the product, and decide whether to use patient protection (MOPP) or operator protection (MOOP).
In either case, the insulation between primary to secondary must meet at least 2 × MOP under and at least 1 × MOP between primary to protective earth (FG) at normal conditions.
The isolation, creepage and insulation requirement for MOPP and MOOP are different and provided in the table below.
| Classifications | Isolation | Creepage / Clearance | Insulation |
| 1 × MOOP | 1500 VAC | 2.5 mm / 2 mm | Básico |
| 2 × MOOP | 3000 VAC | 5 mm / 4 mm | Double |
| 1 × MOPP | 1500 VAC | 4 mm / 2.5 mm | Básico |
| 2 × MOPP | 4000 VAC | 8 mm / 5 mm | Double |
The next article in this series will discuss one of the primary hazards found in medical electrical equipment: corriente de fuga.
Learn more in our white paper — Improving Medical Isolation Transformers with Segmented Core Cap Technology